Select the element whose Lewis symbol is correct takes center stage, this opening passage beckons readers into a world crafted with academic style and authoritative tone, ensuring a reading experience that is both absorbing and distinctly original. Lewis symbols, a cornerstone of chemistry, provide a concise representation of an element’s electron configuration and play a pivotal role in understanding chemical bonding and molecular structures.
As we delve into the intricacies of Lewis symbols, we will explore the rules governing their construction, identify common errors, and establish criteria for evaluating their correctness. Furthermore, we will uncover the practical applications of Lewis symbols, demonstrating their significance in unraveling the complexities of chemical interactions.
The subsequent paragraphs will provide a comprehensive examination of Lewis symbol structure, addressing the placement of electrons, the use of dots and lines, and the periodic trends observed in Lewis symbols. We will also delve into the reasons behind common errors and provide examples to illustrate these pitfalls.
Additionally, a step-by-step procedure will be Artikeld to guide readers in evaluating the correctness of Lewis symbols, ensuring a systematic approach to this essential skill.
Introduction
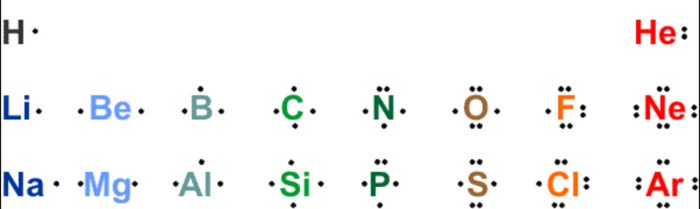
Lewis symbols, also known as electron-dot structures, are a convenient way to represent the valence electrons of an atom or ion. They provide a visual representation of the electron configuration of an element, which is crucial for understanding chemical bonding and predicting molecular structures.
Lewis symbols are particularly useful for main-group elements, which have valence electrons in the outermost energy level. These electrons are responsible for chemical reactions and determine the element’s chemical properties.
Lewis Symbol Structure
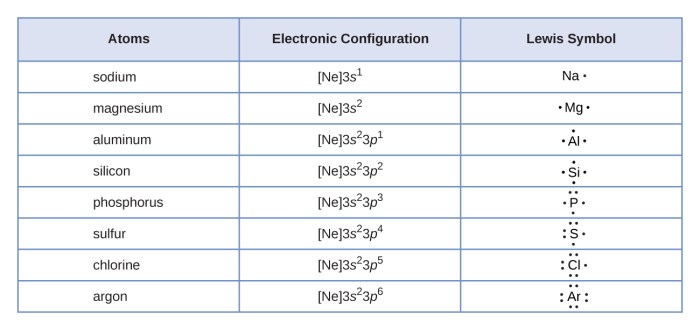
The rules for drawing Lewis symbols are straightforward:
- The symbol of the element is placed in the center.
- The valence electrons are represented by dots placed around the element symbol.
- Each dot represents one valence electron.
- Electrons are arranged in pairs, with unpaired electrons represented by single dots.
- The number of valence electrons is determined by the group number of the element in the periodic table.
For example, the Lewis symbol for hydrogen (H) is a single dot, indicating its one valence electron. The Lewis symbol for oxygen (O) is two dots, indicating its two valence electrons. The Lewis symbol for nitrogen (N) is three dots, indicating its three valence electrons.
Common Errors in Lewis Symbols
Common errors in drawing Lewis symbols include:
- Incorrect number of valence electrons:The number of dots in the Lewis symbol should match the number of valence electrons for the element.
- Incorrect placement of electrons:Valence electrons should be placed in pairs, with unpaired electrons represented by single dots. Lone pairs of electrons should be placed as far apart as possible.
- Incomplete octets:For main-group elements, the Lewis symbol should have a complete octet of electrons, except for hydrogen and helium, which have a duet of electrons.
- Missing charges:Ions have a net charge, which should be indicated by a superscript after the element symbol in the Lewis symbol.
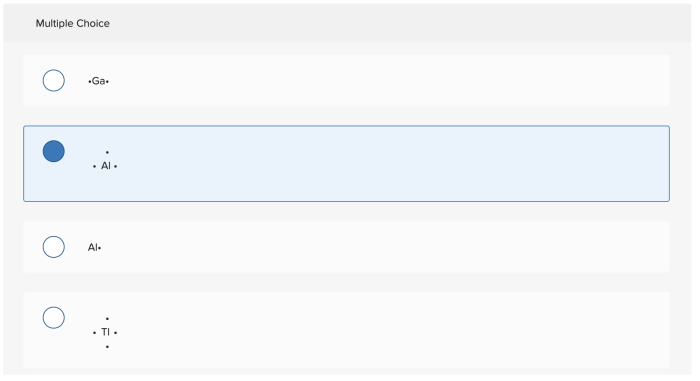
Evaluating Lewis Symbol Correctness: Select The Element Whose Lewis Symbol Is Correct
To evaluate the correctness of a Lewis symbol, follow these steps:
- Count the number of valence electrons:Determine the number of valence electrons for the element based on its group number in the periodic table.
- Check the number of dots:The number of dots in the Lewis symbol should match the number of valence electrons.
- Verify the electron arrangement:Valence electrons should be placed in pairs, with unpaired electrons represented by single dots. Lone pairs of electrons should be placed as far apart as possible.
- Check for complete octets:For main-group elements, the Lewis symbol should have a complete octet of electrons, except for hydrogen and helium, which have a duet of electrons.
- Confirm the charge:If the element is an ion, the Lewis symbol should indicate the net charge by a superscript after the element symbol.
Applications of Lewis Symbols
Lewis symbols have numerous applications in chemistry, including:
- Understanding chemical bonding:Lewis symbols can be used to predict the type of chemical bond that will form between two atoms. For example, atoms with unpaired electrons can form covalent bonds by sharing electrons to achieve a complete octet.
- Predicting molecular structures:Lewis symbols can be used to determine the molecular geometry of a compound. For example, molecules with lone pairs of electrons have different molecular geometries than molecules without lone pairs.
- Explaining chemical reactions:Lewis symbols can be used to illustrate the changes in electron configuration that occur during chemical reactions.
Essential Questionnaire
What is the significance of Lewis symbols in chemistry?
Lewis symbols provide a simplified representation of an element’s electron configuration, enabling chemists to visualize the distribution of valence electrons. This information is crucial for understanding chemical bonding and predicting the behavior of elements in chemical reactions.
How can I identify common errors in Lewis symbols?
Common errors in Lewis symbols include incorrect placement of electrons, violation of the octet rule, and failure to account for lone pairs. By understanding the rules governing Lewis symbol construction, you can avoid these pitfalls and ensure the accuracy of your representations.
What are the applications of Lewis symbols beyond chemical bonding?
Lewis symbols extend their utility beyond chemical bonding to applications such as predicting molecular shapes, understanding acid-base reactions, and visualizing electron transfer processes. Their versatility makes them a valuable tool for chemists across various fields.