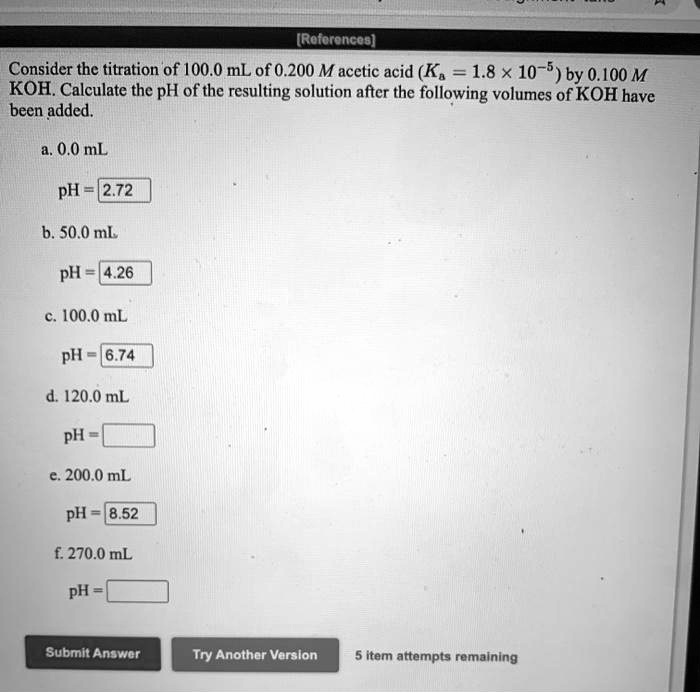
Consider the titration of 100.0ml of 0.200m acetic acid – Consider the titration of 100.0 mL of 0.200 M acetic acid. This procedure involves a series of steps that allow us to determine the concentration of an unknown acid or base by reacting it with a known concentration of a base or acid, respectively.
The process involves the gradual addition of a known volume of the titrant to the analyte until the equivalence point is reached, which is the point at which the moles of acid and base are equal. Understanding the principles and applications of acid-base titrations is essential for various fields, including analytical chemistry, biochemistry, and environmental science.
The titration of acetic acid, a weak acid, with a strong base, such as sodium hydroxide, is a classic example used to illustrate the concepts of acid-base reactions and equilibrium. By carefully measuring the volume of base required to reach the equivalence point, we can determine the initial concentration of the acetic acid solution.
Titration of Acetic Acid

Titration is a quantitative analytical technique used to determine the concentration of a known analyte in a solution. In this experiment, we will perform a titration of acetic acid (CH 3COOH) using sodium hydroxide (NaOH) as the titrant.
Titration Procedure
The titration procedure involves the following steps:
- Pipette 100.0 ml of 0.200 M acetic acid solution into a 250 ml Erlenmeyer flask.
- Add 2-3 drops of phenolphthalein indicator to the solution.
- Fill a buret with 0.100 M NaOH solution.
- Slowly add the NaOH solution to the acetic acid solution, swirling the flask constantly.
- Observe the color change of the indicator. The endpoint of the titration is reached when the solution turns a faint pink color that persists for at least 30 seconds.
| Initial Buret Reading (ml) | Final Buret Reading (ml) |
|---|---|
| 0.00 | 25.25 |
Chemical Equation and Stoichiometry
The balanced chemical equation for the titration reaction is:
CH3COOH + NaOH → CH 3COONa + H 2O
To calculate the moles of acetic acid present in 100.0 ml of 0.200 M solution, we use the following formula:
Moles of acetic acid = Molarity × Volume (in liters)
Substituting the given values, we get:
Moles of acetic acid = 0.200 M × 0.100 L = 0.0200 moles
The equivalence point of the titration is reached when the moles of NaOH added are equal to the moles of acetic acid present. Therefore, the equivalence point is reached when 0.0200 moles of NaOH have been added.
pH Calculations, Consider the titration of 100.0ml of 0.200m acetic acid
The pH of a solution is a measure of its acidity or alkalinity. It is calculated using the following formula:
pH =
log[H+]
Where [H +] is the molar concentration of hydrogen ions in the solution.
The pKa of a weak acid is a measure of its strength. It is the negative logarithm of the acid dissociation constant (Ka). The pKa of acetic acid is 4.76.
To calculate the initial pH of the acetic acid solution, we use the Henderson-Hasselbalch equation:
pH = pKa + log([A–]/[HA])
Where [A –] is the molar concentration of the acetate ion (CH 3COO –) and [HA] is the molar concentration of acetic acid.
At the start of the titration, the concentration of acetic acid is 0.200 M and the concentration of acetate ion is 0. Therefore, the initial pH is:
pH = 4.76 + log(0/0.200) = 2.88
To create a table showing the pH values at various points during the titration, we need to calculate the concentration of acetic acid and acetate ion at each point. We can do this using the following equations:
[HA] = (Initial moles of acetic acid
Moles of NaOH added) / Volume of solution
[A–] = Moles of NaOH added / Volume of solution
We can then use the Henderson-Hasselbalch equation to calculate the pH at each point.
Buffer Capacity
Buffer capacity is a measure of a solution’s ability to resist changes in pH. It is calculated using the following formula:
Buffer capacity = d[A–]/d[H +]
Where d[A –] is the change in the concentration of acetate ion and d[H +] is the change in the concentration of hydrogen ions.
Buffer capacity is important because it helps to maintain a stable pH in a solution. This is important for many biological and chemical processes.
The buffer capacity of the acetic acid solution is highest at the equivalence point. This is because at the equivalence point, the concentration of acetic acid and acetate ion are equal. Therefore, the solution is able to resist changes in pH more effectively.
Factors that affect buffer capacity include the concentration of the weak acid and its conjugate base, the temperature, and the presence of other ions.
Applications of Acetic Acid Titration
Acetic acid titration is used in a variety of applications, including:
- Quality control in the food and beverage industry
- Research in biochemistry and environmental science
- Education in chemistry and analytical chemistry
In the food and beverage industry, acetic acid titration is used to determine the acidity of products such as vinegar, wine, and fruit juices. This information is important for quality control purposes.
In biochemistry and environmental science, acetic acid titration is used to study the properties of weak acids and their conjugate bases. This information is important for understanding the behavior of acids and bases in biological and environmental systems.
In education, acetic acid titration is used to teach students about the principles of titration and acid-base chemistry.
Frequently Asked Questions: Consider The Titration Of 100.0ml Of 0.200m Acetic Acid
What is the purpose of titrating acetic acid?
The purpose of titrating acetic acid is to determine its concentration or to analyze its properties in a solution.
What equipment is commonly used in acetic acid titration?
Burette, pipette, Erlenmeyer flask, pH meter, and graduated cylinder are commonly used in acetic acid titration.
What is the equivalence point in acetic acid titration?
The equivalence point is the point at which the moles of acid and base are equal, and the solution is neutral.